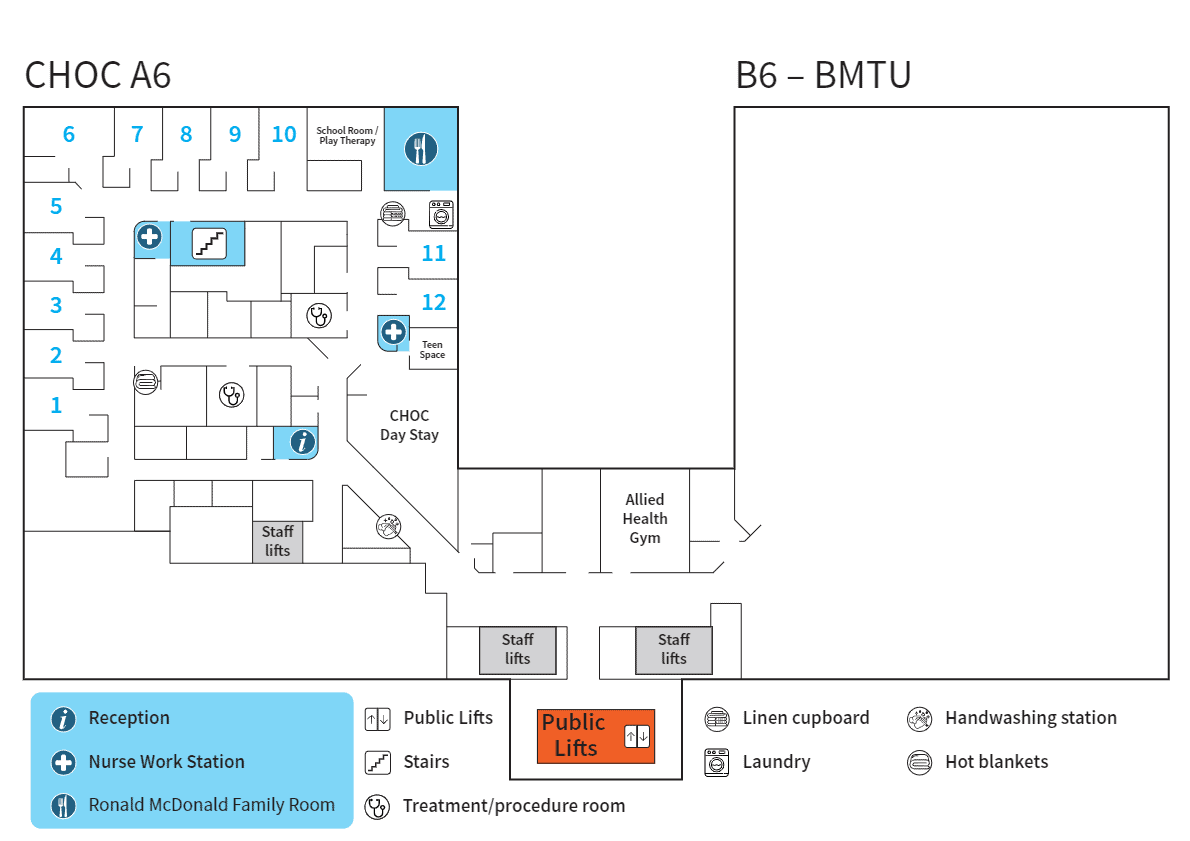
Consultants
Consultant Oncologists are essentially in charge of your child’s medical care. Any decision about their treatment, medication, interventions or stay in hospital will be made in consultation with the family/whanau and other members of the medical team as is required by your child’s specific case. Any major decisions about your child’s health will be considered firstly and thoroughly by their consultant. It is with the consultant that you should raise any major concerns about changes in your child’s health during their treatment.
Registrars
The day-to-day medical care for children is provided by two registrars. A Paediatric Registrar is a doctor who is undertaking specialist training in Child Health. We may also have a registrar who is completing their Oncology or Haematology Fellowship who has a particular interest in paediatric oncology and may go on to become a Paediatric Oncologist/Haematologist. They spend one year in CHOC.
Every Monday, Tuesday and Friday the Consultants, Fellow, Registrar and Nurse Practitioner will do a full ward-round and see your child. Every other day your child will be seen by the Fellow, Registrar or Nurse Practitioner.
Charge Nurse Manager
The Charge Nurse Manager is responsible for the day-to-day running of the ward and is also available to discuss any concerns you may have on the quality of your child’s care. The Charge Nurse Manager works 8.00am to 4.30pm Monday–Friday and her office is situated at the end of the left corridor opposite day stay unit.
Senior Nurses
Nurse Practitioner
The Nurse Practitioner is an extended nursing role which combines advanced nursing expertise and some roles usually undertaken by medical staff. She attends the ward-rounds and may also examine your child on the days that the ward-round does not occur.
Clinical Nurses Specialists
Clinical Nurse Specialists (CNS) are senior nurses with responsibility for a specific area of nursing, such shared care. They have extended knowledge and expertise in paediatric oncology.
Shared Care
The shared care service has responsibility of coordinating treatment and care for all families who live outside Christchurch. They liaise with the other regions to ensure your child’s treatment is of a consistently high standard.
LEAP Coordinator
Our LEAP (Late Effects Assessment Programme) coordinator has the role of organising follow up appointments for families post the end of treatment.
Nurses
Nurses are responsible for the day-to-day, immediate care of your child. Nurses are allocated according to skill mix and patient acuity. The nurses work eight-hour shifts. Nursing handover times at the end of each shift are:
| Morning Shift: 6.45am |
Afternoon Shift: 2.30pm |
Night Shift: 10.30pm |
To ensure continuity of care for your child a bedside handover will be performed. We aim to respect your wishes regarding what information is discussed in front of your child. Please let your nurse know of any concerns or questions you may have so they can inform the appropriate staff. Your nurse will complete a care plan in conjunction with you to ensure continuity of care through all shifts.
Hospital Aides
Hospital Aides keep the ward well stocked with supplies and attempt to keep CHOC tidy. They wear a maroon-coloured uniform. Sometimes Hospital Aides are available to keep an eye on your child for a short period, such as when you are having a meeting with one of the consultants or need a shower. They also answer bells to assist the nurses if the ward is busy. Let your nurse know if you need the hospital aide to provide you some respite to organise a convenient time.
Administration Assistant
The Ward Clerk deals with all clerical duties on the ward, including patient enquiries, the completion of forms and correspondence regarding the patient.
Ward Cleaner
Every day the Ward Cleaner will clean your room. On Wednesday’s and Sunday’s, the cleaner will also pull the furniture away from the walls to ensure any dust is removed from behind the furniture. Please assist them by picking up your personal belongings off the floor. Not involved in direct patient care, if any queries or concerns please discuss with your nurse.
Kitchen Aide
The Kitchen Aide delivers meals at set times through the day. Not involved in direct patient care, if any concerns or questions please discuss with you nurse.
Hospital Play Specialists
The Hospital Play Specialists set up the CHOC activity room and cater for all children, including teenagers. Some of your child’s treatment can be difficult to tolerate at times and can be upsetting for you and your family. The Play Specialist can help provide support and distraction during medical procedures and provide toys and art supplies to relieve boredom. Additionally, parents and children can access educational resources to take back to their room, including wipeable resources if they are in isolation.
Social Work
The social work team provides an ongoing service to all families who come to Christchurch Hospital. They recognise that families who are caring for a sick child experience considerable disruption to their lives.
The Social Workers in CHOC are available to provide emotional and practical support for you and your family, including providing information about financial supports. The social workers can link you up with social support systems in your local community including transport/hospital parking assistance, accommodation, counselling services, interpreters and community support groups such as Child Cancer Foundation.
Psychologist
You probably have lots of questions and feelings to sort out in your head as you adjust to changes in your life. The people who care for you may be very emotional too. Having feelings of shock, anger and fear are all normal. The Psychologist’s job is to help you and your family to cope and adjust. They will talk to you about ways to manage your emotional reactions and behaviour in a healthy way. If you wish for support at any time please let your nurse know and she will contact a psychologist on your behalf.
Pharmacist
There may be a number of different medicines your child needs to take and it is very important they are taken as prescribed. In addition to the nursing staff, the pharmacist can help you and your family understand what each drug is for, and inform you of the common side effects and special precautions you need to take.
A special yellow medication card will be written for you and your family, with all the information you need about the drugs you will take when you are at home. It is a good idea for you and/or your parent/caregiver to take the yellow medication card wherever you go. You will have a record of all the medications you are taking, in case of an emergency. Your prescription may change as you progress through your treatment time and your yellow medication card will need adjusting from time to time.
Māori Health Service
Ngā Ratonga Hauroa Māori, Christchurch Hospital and Christchurch Women’s Hospital will provide āwhina/support to tūroro/patient and whānau/family while they are using the hospital. Our doors are open to all cultures.
Our role is to assist tūroro through the hospital system and make the journey as smooth as possible. We are available to assist you:
- with your permission, by being present at appointments and consultations to listen to you
- to contact whānau for you at your request
- with providing community services information that may be of use when you are discharged from the hospital
- with low cost accommodation (if available) for those who have travelled out of the Christchurch area
To contact us (03) 364 0640 ext.86160.
Physio
Our Physio Therapists help children to achieve their optimal physical development. They have specialist knowledge in the movement and development to help with possible physical changes that may occur throughout your child’s treatment, whether it is disease or treatment related.
Occupational Therapy
Our Occupational Therapists (OT) enable children to participate fully in the activities of life, covering self-care, productivity and play or care.
Dentist
Throughout your child’s treatment they will be referred to the dentist for education to ensure adequate oral health, as some treatments can have a large effect on teeth, gums and oral mucosa.
Dietitian
All children in CHOC will be referred to the dietician on their first admission. The Dietitian will monitor your child’s food intake and weight and prescribe nutritional supplements to help maintain a healthy weight and energy level. Medication can alter appetite and the way some foods taste. It is very important that your child gets the correct amount of nutrition they need throughout treatment. The Dietitian can also provide ideas on tasty meal options and is also available on request to discuss any nutritional questions about your child.
Questions are best asked about your child’s condition and treatment when the doctors are reviewing your child on ward round in the mornings. We encourage you to ask questions so that you can gather a good understanding of your child’s condition and treatment required. If you are wanting to request to see a member of staff whether that be your Oncologist, Dietitian , Physio etc then please speak with your nurse who can liaise with them to arrange a time that suits you both.
Chaplains
 Chaplains respond to the spiritual, emotional and pastoral needs of patients and their whanau/family. We do this by being a ‘presence’, by listening, affirming other cultures and religions, and by prayers, blessings, encouragement and support. their clinical condition.
Chaplains respond to the spiritual, emotional and pastoral needs of patients and their whanau/family. We do this by being a ‘presence’, by listening, affirming other cultures and religions, and by prayers, blessings, encouragement and support. their clinical condition.
Chaplains are available office hours on site Monday – Friday and after hours for emergencies via the operator for the on-call chaplain.
Rev. Moega Lasei Ph 021 712 895 / Ext 86358
Sheila Mark, Chaplain Ph 021 569 604 / Ext 89555
Pastor Donna Reid Ph 021 198 6927 / Ext 89555
Rev. Helen Gray, Maori Chaplain Ph 021 730 457 / Ext 86372
Rev. Alexa Evenden, Chaplain – Women’s and Children’s Health Ph 021 708 853 / Ext 85722
Angela McCormick, Roman Catholic Chaplain Ph 021 702 378 / Ext 89554
Research Activity Team
The team at the Children's Haematology Oncology Centre (CHOC) belong to several international study groups:
- Children's Oncology Group (COG, USA),
- International Society of Paediatric Oncology (SIOP, worldwide)
- Australia and New Zealand Children's Haematology Oncology Group (ANZCHOG)
These groups develop treatment trials for most paediatric oncology conditions, often comparing the best-known treatment with a subtle variation. During a trial, treatment and associated side-effects and long-term outcome are closely monitored. Clinical Research Associates/ Coordinators (CRAs) on CHOC manage the study-related activities.
If a child, adolescent or young adult is considered eligible for a clinical trial, the details of this will be discussed. The parents and patient (if appropriate for age) will be given an Information Sheet and Consent Form relating to the particular trial. Entry onto a clinical trial is voluntary.
All parents will be given an Information Sheet and Consent form about tissue banking for them to consider. The Tissue Bank ensures that tissue, in excess of that required for diagnosis, already taken or removed at surgery can be saved and kept to allow future research with the potential to further advance knowledge about the condition.
CHOC participates in the New Zealand Child Cancer Registry, this is funded by the Ministry of Health to ensure accurate data is kept about the type and incidence of Childhood Cancers in New Zealand.
As a unit involved in research, we are required to ensure that the Health and Disability Ethics Committee (HDEC) has approved all studies before they are opened at our centre. HDEC reviews and approves all aspects of the study, including the Patient Information Sheets and Consent forms.
Late Effects Assessment Programme (LEAP) Team
What is LEAP?
LEAP stands for Late Effects Assessment Programme. It is a Te Whatu Ora service providing long term follow up for survivors of childhood cancer.
The New Zealand Cancer Control Strategy, Action Plan 2005-2010, identified the need to "ensure all survivors of childhood and adolescent cancer receive timely and ongoing support and rehabilitation, including the identification of, and intervention in, late effects" (Goal 4, objective 3, phase 1).
It is estimated that around half of child cancer survivors will have at least one significant problem related to their treatment, for example problems with growth and development, learning and thinking skills, hearing loss, heart damage or fertility.
In response to the Cancer Control Strategy, with initial funding from the Ministry of Health, LEAP commenced as a national programme in 2006, in the three cancer centres for New Zealand (Auckland, Wellington, and Christchurch).
What does LEAP provide?
Long term follow up clinics – multi-disciplinary clinics and nurse-led clinics across the South Island
Neuropsychological assessments (including psychological adjustment, learning and thinking)
Psychosocial assessment and interventions (including therapy sessions, referrals to other agencies)
Adolescent transition to primary health care
Individual treatment summary, known as a "Health Passport" that provides risk based guidelines. A copy is given to the patient and General Practitioner
A national database that incorporates the New Zealand Children's Cancer Register (NZCCR) with LEAP data, and the ability to output health passports for patients and their health care providers
How do patients enter LEAP?
Clients already under the care of the paediatric oncologists are transferred into LEAP at the appropriate time – once treatment and disease surveillance is considered sufficient (from 2-5 years, dependant on the cancer). LEAP also takes referrals from the adult oncology service and GP's for clients who have previously left paediatric care but now require late effects follow up. Clients can even refer themselves (or their child) by contacting the LEAP team directly.
Who is the LEAP team?
The core team comprises:
Paediatric Oncologists
Dr Amanda Lyver
Dr Siobhan Cross
Dr Tristan Pettit
Dr Andrew Dodgshun
Dr Awras Majeed
Senior Clinical Psychologists
Shannae Kean and Kate Spill
Clinical Nurse Specialist/LEAP Coordinator
Mikaela Eder
Routines
Generally, it’s better to maintain normal household schedules while in hospital, but this is not always possible due to a child’s medication, treatment or effects. If you would like support from staff to encourage your child to maintain their routines, please discuss with your nurse.
On admission
Your child and family/whānau will be introduced to your nurse and orientated to the ward. Information will be given to you regarding your child’s care and treatment and the results discussed with you. Please feel free to discuss with the nursing and/or medical staff any queries or concerns you may have during your stay. Your belongings are kept on the ward at your own risk. Please keep your valuables with you at all times.
On day of discharge
We will provide you with discharge letters, scripts, instructions and any relevant information regarding your child’s follow up. Please notify your nurse when leaving. Please check your room before leaving to ensure you have taken all your belongings, including checking sockets and storage unit.
Food
A menu is circulated each morning for you and your child to choose what will be served for lunch and dinner. Help yourself to cereal, toast, hot drinks and fruit, available in the kitchen. However, if you want porridge you may choose that option on the menu. Please leave your completed form on your bedside table, or with your nurse.
If your child is not eating due to nausea or the effects of treatment, parents can help themselves to the hospital meal provided. Meals are only provided by the hospital for patients and breastfeeding mothers.
Family Kitchen
The kitchen is located next to the playroom in the CHOC Day Stay Unit and is open 24/7. You will find some snacks for yourself and your child here, donated by the Child Cancer Foundation and Ronald McDonald, but please be mindful this is for all families.
If you wish to bring in your own food, please store it in a labelled container in the fridge, freezer or drawer. Remember to take all your food home on discharge as we have very limited space. Also, please ensure food has not expired or past its best to use dates.
NOTE// No alcohol is permitted within the hospital.
Cooking facilities include:
- an oven,
- stove top and
- microwave.
NOTE// It is very important to keep the kitchen area clean at all times. Please remember a lot of our children are susceptible to infections.
The hospital has a hot drink policy that requires everyone to use the cups provided and ensure the lid is secure to prevent spillage of hot fluids.
We encourage families and children to eat their meals in the dining area of the kitchen to establish a normal daily routine, as you would at home for your child. This also decreases the infection risk of having food within your child’s room.
Infectious patients and family members are not permitted to use the kitchen area. Please be mindful, our CHOC children are susceptible to infections, thank you.
Other meals are not provided, except in special circumstances in which case the caregiver should consult the Charge Nurse or Social Worker to see if they qualify for meal vouchers.




 Chaplains respond to the spiritual, emotional and pastoral needs of patients and their whanau/family. We do this by being a ‘presence’, by listening, affirming other cultures and religions, and by prayers, blessings, encouragement and support. their clinical condition.
Chaplains respond to the spiritual, emotional and pastoral needs of patients and their whanau/family. We do this by being a ‘presence’, by listening, affirming other cultures and religions, and by prayers, blessings, encouragement and support. their clinical condition.