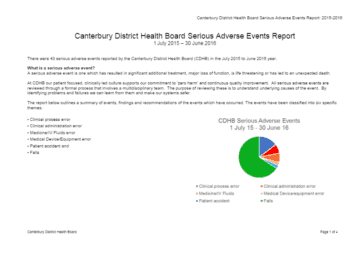
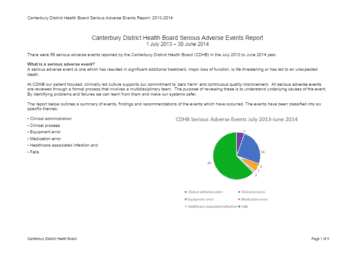
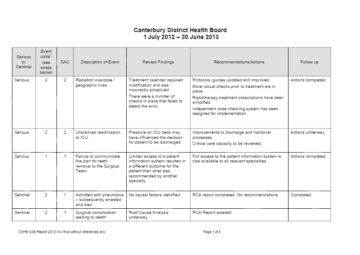
I'm not well, where do I go?
Health advice 24/7
Call your GP team to talk to a health professional
Pharmacy
Diarrhoea, colds, hayfever, skin complaints
Specific care
Mental health, injuries, flu, dental, child, pregnancy
General practice
Non-urgent health issues that aren’t improving
Urgent care 24/7
Bad sprains, minor head injuries, stomach pain
Emergency Department
Serious accidents, chest pains, stroke call 111